Colorant is the generic term for the material that absorbs certain wavelengths of light, allowing the rest to reflect or transmit through an object. As we dive deeper into this module, we will review the interaction between light and matter on a molecular level to understand how more complicated colorants, such as fluorescents, work. For now, we start with standard dyes and pigments.
As technology advances, the line between dyes and pigments continues to blur, but generally speaking, dyes are colorants that are soluble (in water, oil, or solvents), while pigments are particles that are insoluble. This means that dyes typically dissolve into the material being produced, forming a molecular bond directly, while pigments typically require a binding agent to hold them in place. Another distinction is that dyes are mostly organic. Pigments have traditionally been inorganic, though organic pigments are on the rise.
Because dyes dissolve into the material being produced, they tend to provide better transmission, making them easier to use for many transparent applications. But they also have a tendency to leach out. Pigment molecules tend to be more stable, making them more durable against water, wind, sunlight, heat, etc. This typically makes pigments the ideal choice when long term durability is required, but it also poses unique challenges when high transmission is needed (like with sign sheeting). Pigments could be used to make translucent materials that are also durable, but a binding agent (which encases the pigment) with a similar refractive index as the pigment is required. The aggregation of the pigment particles also needs to be tightly controlled.
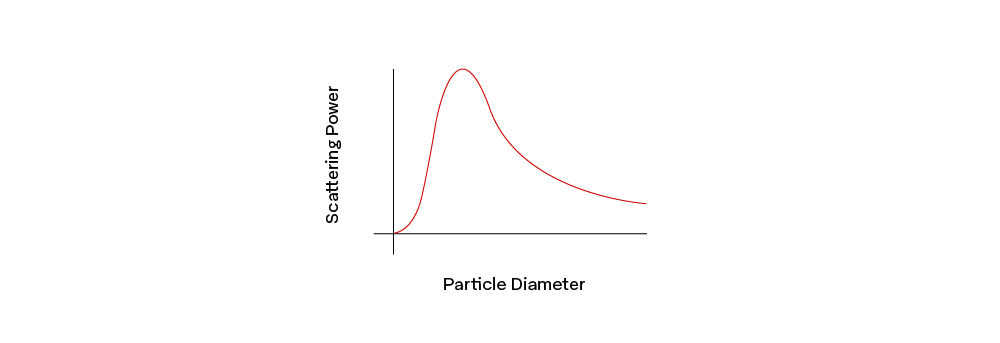
As discussed in Chapter 1 of our Retroreflectivity module, when light goes through an object, it is said to be transmitted, and the material of the object is considered to be transparent. The object’s optical density will impact its refractive index (n), which determines light's velocity as it passes through the object. As light passes from one transparent medium (such as air) to another with a different refractive index (such as acrylic), it will bend. As it does, a small amount of light will be scattered–once when the light enters the new medium and again as it exits. This is why using a binding agent with a similar refractive index as the pigment particles limits the amount of light scattering.
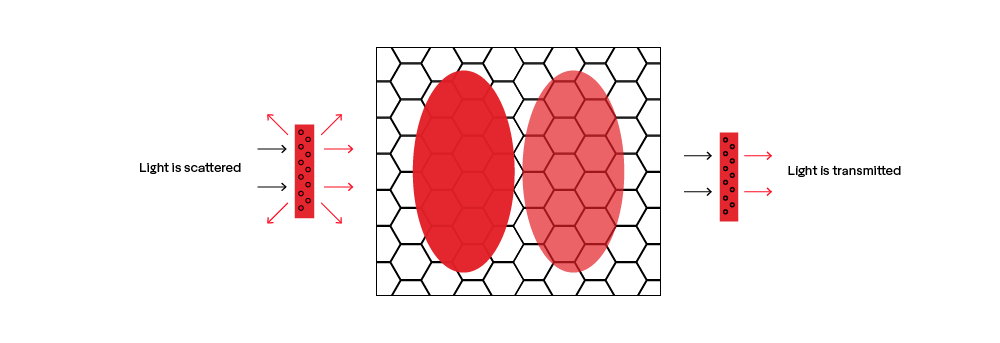
In retroreflective sheeting, this happens on an almost molecular level with each pigment, but the best way to show the effect is by placing glass in olive oil. Because glass and olive oil have the same refractive index, light is not bent or scattered as it passes through them, making the glass invisible. The same is true when the correct combination of pigments and binding agents are used.
As a transparent material begins to scatter more light, either at the surface (Chapter 1 of Module 1) or because of particles within it, it becomes translucent. If the scattering is so intense that no light passes through, the material is considered to be opaque.
A material's transparency and the way it scatters light could impact the perceived color because color is the result of the combination of wavelengths entering the eyes and their relative intensity. In the figure above, for example, we can see that a translucent red may appear lighter (or more pink) than an opaque red when both are applied to a white material. Likewise, as shown below, the color of a glossy surface may appear more saturated (less white) than a textured surface. In all instances, the colorant used could be the same, but the chemistry and physical properties surrounding the colorant will impact how it is perceived.

One way to quantify a colorant is to measure its Reflectance Factor (RF) in the case of opaque material or its Spectral Transmittance (ST) in the case of transparent materials.
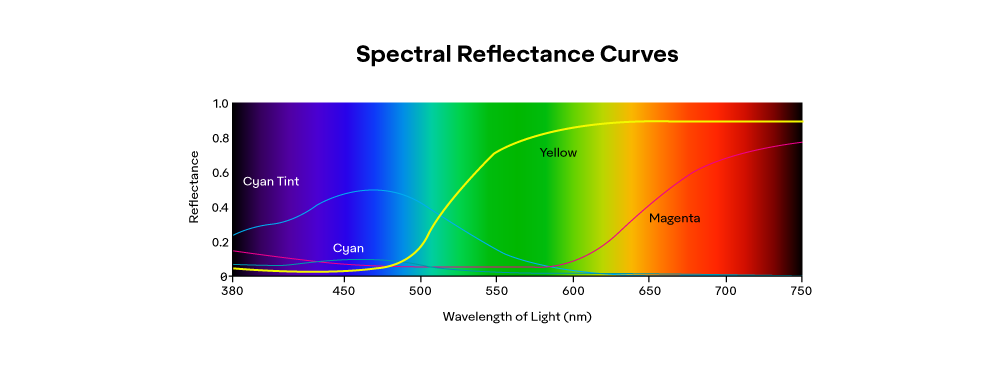
If all of the visible light is absorbed, we get black. If all of the visible light transmits, the object would be clear or colorless. The color white is usually the result of clear particles scattering light (like in a cloud), so our brain senses the resulting impulse as white.