Typical human vision works thanks to photoreceptors in the eye, known as rods and cones. Energy from visible light triggers a chemical reaction within these photoreceptors, which converts the energy into an electrical impulse to the brain. Rods help us see in low-light scenarios, such as moonlight, while cones help produce color in well-lit conditions greater than 10 cd/m^2.
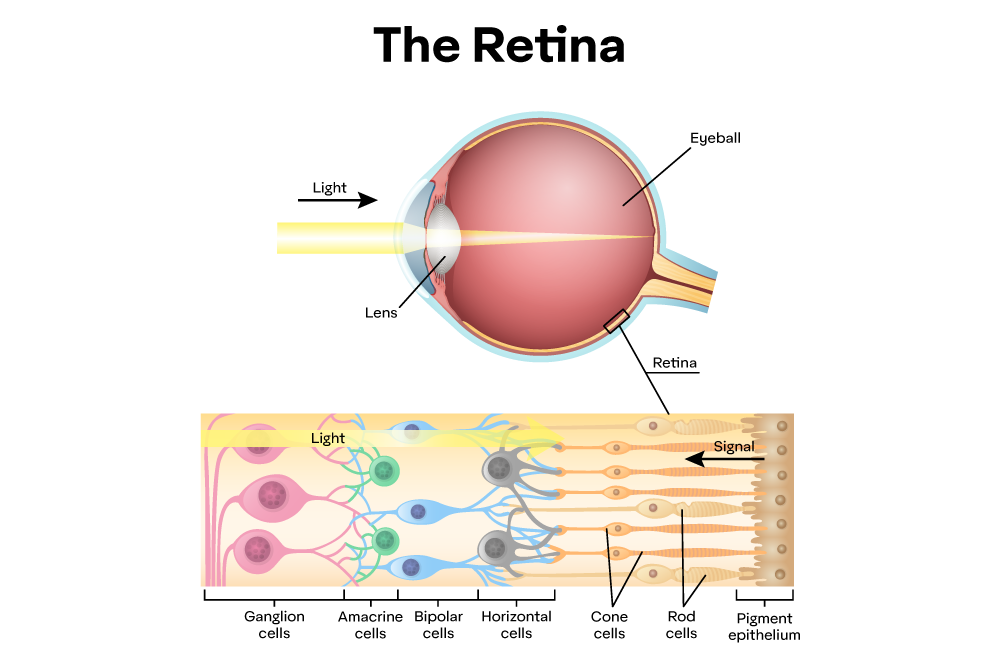
Normally people have trichromatic vision, meaning there are three types of cones: Red (R), Green (G), and Blue (B). Their labels are more of a convention and come from their ability to absorb relatively Long (L), Medium (M), and Short (S) electromagnetic wavelengths within the visible spectrum, respectively:
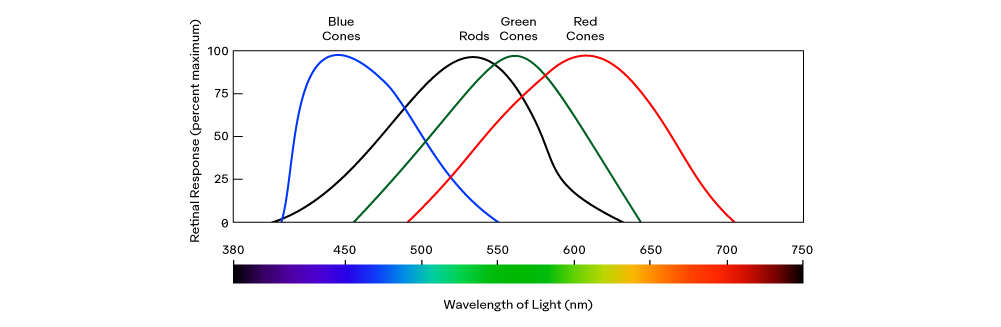
The difference in the sensitivity curves of the three types of cones is how we interpret wavelengths of visible light as a specific hue, such as “blue" or “yellow." For example, electromagnetic waves of about 650 nm to 670 nm will excite R while very weakly exciting G or B cones; our brains perceive the ratio between R to G to B as the sensation of yellow.
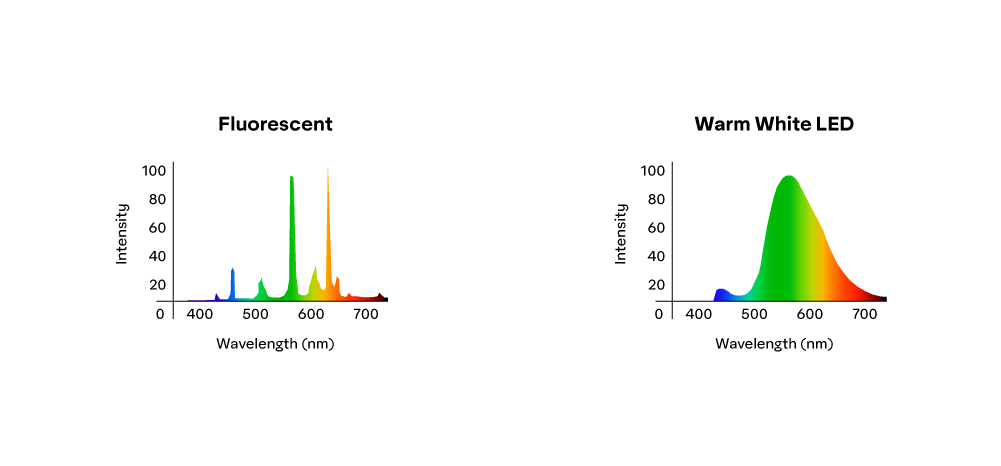
Signals from the different types of cones (and rods) are merged as they move through the visual pathway (towards the ganglion cells), so we cannot actually distinguish between red and green light activating the Red and Green cones simultaneously or yellow light doing the same. In both instances, we see yellow. It is also why the two light sources in the illustration below might appear to have a similar "warm white" color, despite having different spectral power distributions:
 By blending wavelengths, our visual systems are also capable of making up colors, not in the rainbow; these are non-spectral colors. Some examples are the colors purple and magenta, which are produced when the Blue and Red cones are strongly activated while the Green cones are weakly activated. Another example would be white, which is the result of all three cone types being activated to a similar degree.
By blending wavelengths, our visual systems are also capable of making up colors, not in the rainbow; these are non-spectral colors. Some examples are the colors purple and magenta, which are produced when the Blue and Red cones are strongly activated while the Green cones are weakly activated. Another example would be white, which is the result of all three cone types being activated to a similar degree.
Chroma or "purity” or “saturation" of a hue is based on the region of sensitivity curves activated. The fluorescent and warm white led lights depicted in the graphs above, activate many cones, so we categorize them as white (or "warm white”) bulbs. As such, they don't have a specific spectral color, and so their “purity" or chroma is low.
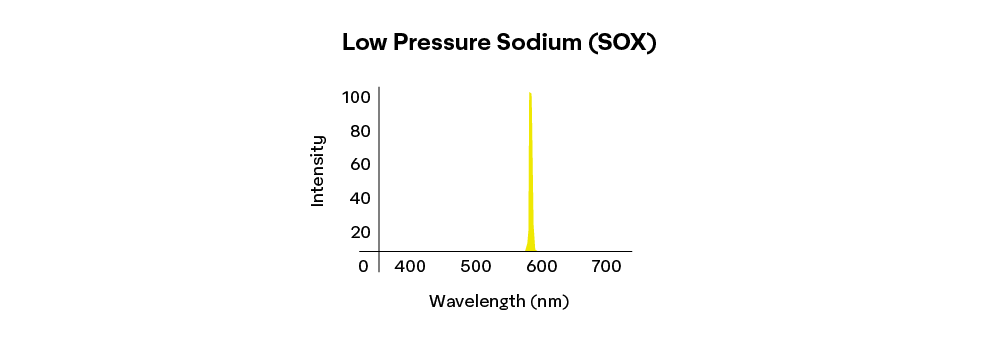
Conversely, lights with a narrow spectral bandwidth are known as monochromatic light. The low-pressure sodium light illustrated below, for example, will activate a relatively narrow section of the sensitivity curves of the eye and appear a saturated yellow; so it will appear as a highly saturated, or high chroma, yellow.
Not all wavelengths are perceived the same, though. The figure below, formally known as the CIE Photopic Spectral Luminous Efficiency Function V(𝝀), or simply the photopic response of the eye, shows the relative response of the average eye to the various wavelengths across the visual spectrum. It shows a range of about 400 nm to 700 nm, peaking at about 555 nm. The result is that colors near the middle of the visible spectrum appear brighter than blues and reds. Additionally, the "just-noticeable difference" in wavelength varies from about 1 nm in the blue-green and yellow regions versus about 10 nm or more for reds and blues.
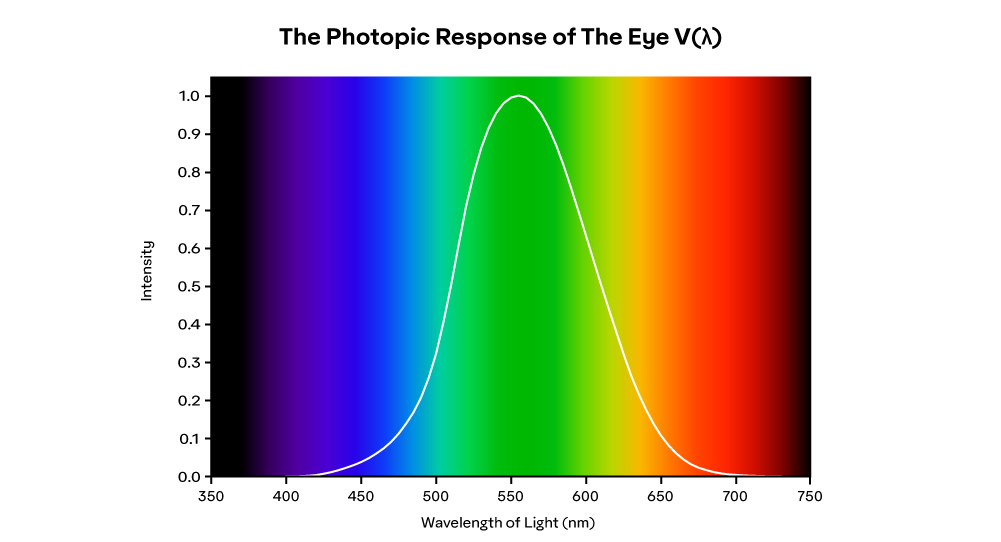
Brightness can be defined as the intensity of illumination. However, brightness is a relative measure for multiple reasons. As shown in the photopic response curve above, our eyes do not have the same level of sensitivity to all wavelengths. Additionally, even though our eyes can operate from bright daylight (about 50,000 to 100,000 lx) to moonlight (about 10 mlx), our pupils adjust to different intensities of light to maintain a desired level of light entering the eye (about a 100:1 contrast ratio). This adds another dimension to how color is perceived and gives us the ability to see pink, brown, and black, and notice when something is fluorescent. Topics that we will discuss in subsequent chapters.



 By blending wavelengths, our visual systems are also capable of making up colors, not in the rainbow; these are non-spectral colors. Some examples are the colors purple and magenta, which are produced when the Blue and Red cones are strongly activated while the Green cones are weakly activated. Another example would be white, which is the result of all three cone types being activated to a similar degree.
By blending wavelengths, our visual systems are also capable of making up colors, not in the rainbow; these are non-spectral colors. Some examples are the colors purple and magenta, which are produced when the Blue and Red cones are strongly activated while the Green cones are weakly activated. Another example would be white, which is the result of all three cone types being activated to a similar degree.