As discussed in Chapter 4, most objects get their color from the pigments and dyes used in their production, which absorb certain wavelengths of light, allowing the rest to reflect or transmit through an object. This happens on an atomic level, where the atoms and molecules they form have their own characteristic wavelengths. When photons with a matching wavelength interact with certain sections of these molecules, the photon is absorbed and the molecule moves from its ground state to an excited state. The section of the molecule responsible for color is known as its chromophore.
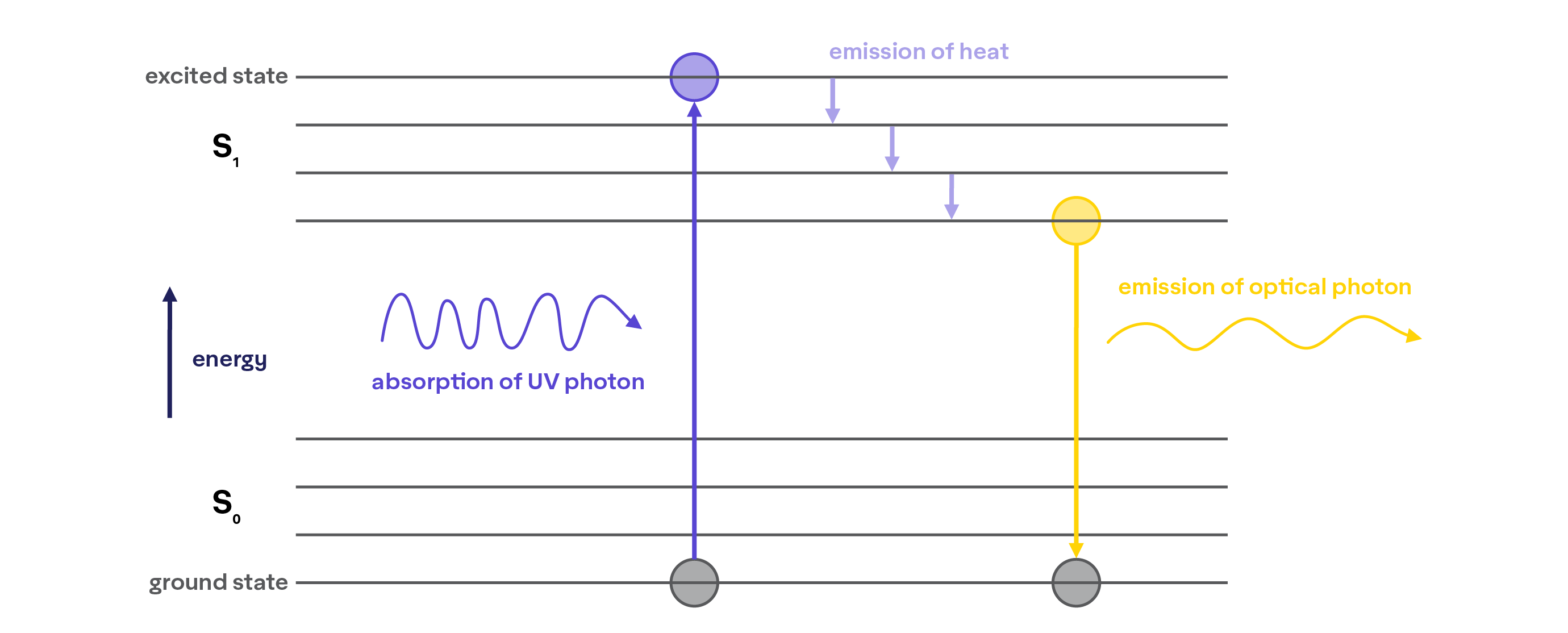
With most standard colorants, the excited molecules twist and turn and “transition” the extra energy absorbed from the photon as infrared light, i.e.: heat:
However, there are also molecular structures, called fluorophores, that can absorb ultraviolet or near UV light and emit it as visible light, producing fluorescent colors.
From a molecular perspective, fluorophores work similarly to chromophores in that their electrons absorb light energy and are pushed to an excited state. However, instead of turning all of the absorbed energy into heat, enough is transitioned so that the energy gap (ΔE) between the excited state and the ground state of the electron corresponds to a wavelength within the visible spectrum. As the electron then jumps back to its ground state, it emits light within the visible spectrum.
This creates a fluorescent effect because, as discussed in Chapter 4, we process color by subconsciously taking the highest reflection factor in a given scene and calibrating that as white (known as “scene white”). We then calculate the expected brightness of all other colors in that scene relative to what we believe white (the brightest color in that scene) should look like. And as discussed in Chapter 2, the difference in the sensitivity curves of the three types of cones is how we interpret wavelengths of visible light as a specific hue, such as “blue” or “yellow."
In the case of fluorescent colors, such as fluorescent yellow, our cones are sending a signal corresponding with a yellow color. At the same time, our brain expects yellow to have a certain brightness based on context and our personal experiences. The extra intensity created by the fluorescent colorants, is seen as a brighter and a more conspicuous yellow. As the ratio of visible light to ultraviolet light changes throughout the day or due to weather conditions, such as dawn, dusk, or cloudy days, so does the perceived glow.